Texas Congressman Lloyd Doggett said he will look into addressing growing concerns over medical device dangers and testing nationally. 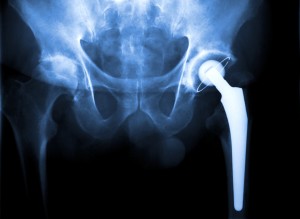
This comes after KVUE-TV did an investigative piece involving a Texas woman who has had numerous serious issues with her hip implants. The woman was born without sockets and her doctors recommended implants—the Pinnacle with Ultamet liner, manufactured by Johnson & Johnson’s Depuy.
After surgery, the woman suffered severe pain and was having issues with her face, including boil breakouts on her skin. She also suffered memory loss.
She eventually discovered online, through searching European records, that many people with similar hip implants suffered from metal poisoning as their devices failed and denigrated inside their bodies.
Sadly, the woman did not find much government-backed research or information, because the U.S. has no medical device registry.
Eventually, she was made aware that there were ongoing lawsuits over the device failures in the U.S. and saw documents that showed:
Depuy’s development team warned company leaders of issues with metal-on-metal implants in 1997 before patients started getting Pinnacle with Ultamet liner implants.
Johnson and Johnson’s Depuy currently faces more than 10,000 lawsuits across the United States. The woman eventually had to have her implants surgically removed. She says she still suffers consequences including soft tissue damage.
How Are Medical Devices in the U.S. Approved for Use?
The woman also discovered how poorly medical devices in the U.S. are regulated and approved.
The U.S. Food and Drug Administration (FDA) has two methods for medical device approval: 510(k) or petition for premarket submission (PMA). Most companies choose to go through the 510(k) route because it does not involve much scientific and regulatory review.
Through the 510(k) process, devices can be marketed if they fall into previously existing classification categories. What happens in many cases is that devices are placed and submitted into classifications that already exist, so they are not scrutinized. Unlike medical drugs, devices that fall into categories are often not tested in trials.
In response, Doggett said he would look into sponsoring legislation to address the lack of medical device oversight.
“The patient ought not to be the guinea pig here and that seems to be what has happened,” he said. “There needs to be better testing and a thorough registry so that physicians and patients can find out specifically what risk they are assuming when they get an implant.”
Doggett is the chairman of the Health Subcommittee on the House Ways & Means Committee.
Can You Hold a Defective Medical Device Manufacturer Liable for Injuries?
Defective medical device manufacturers must be held liable when they injure or kill someone. If you or a loved one has been injured by a faulty medical device like a hip or knee implant, contact our Texas defective medical device attorneys for a no-cost consultation.
